-
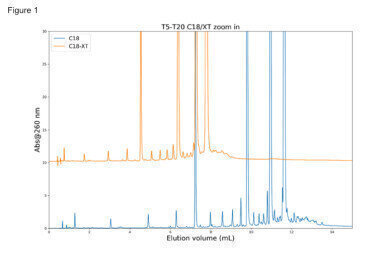 Figure 1: Kromasil Classic C18 compared with a Kromasil EternityXT C18, using TEAA, 50 mM, as ion-paring agent
Figure 1: Kromasil Classic C18 compared with a Kromasil EternityXT C18, using TEAA, 50 mM, as ion-paring agent -
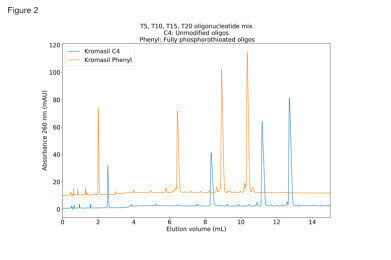 Figure 2: chromatograms show the effect of using TBuAA as ion-pairing agent, to achieve sharp peaks even with PS modified oligos
Figure 2: chromatograms show the effect of using TBuAA as ion-pairing agent, to achieve sharp peaks even with PS modified oligos -
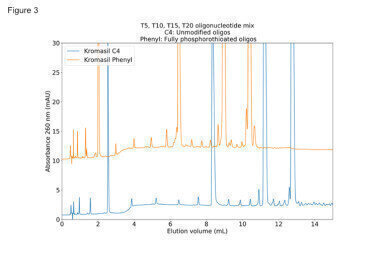 Figure 3: chromatograms (zoomed-in portion of figure 2) that even with the phosphorothioated oligos it is possible to obtain very sharp peaks, despite the enormous number of different diastereomers.
Figure 3: chromatograms (zoomed-in portion of figure 2) that even with the phosphorothioated oligos it is possible to obtain very sharp peaks, despite the enormous number of different diastereomers.
HPLC, UHPLC
Oligonucleotide separations with Kromasil RP phases
Dec 12 2018
Therapeutic oligonucleotides (oligos) represent an important area of research in the pharmaceutical industry today. These drug candidates are typically 8-50 nucleotides long, and contain single stranded DNA or RNA. Oligonucleotides are typically analysed using ion-pairing chromatography, and the aim of the present work was to investigate the influence of type of stationary phase, and type of ion-pairing agent. The presented data is part of a bigger study, that will be published shortly.
The oligonucleotides used in the study were desalted and lyophilized oligos from the series T5, T10, T15, and T20, i.e. they contain only a single type of base: thymine. The intermediate n-mers are present as impurities. The columns are all 150 x 3.0 mm, 2.5 μm particle size, and an acetonitrile gradient is applied.
Figure 1 shows a Kromasil Classic C18 compared with a Kromasil EternityXT C18, using TEAA, 50 mM, as ion-paring agent. It can be seen that the Classic C18 exhibits more retention, while the EternityXT shows better selectivity.
A problem associated with oligonucleotides is that they are easily degraded via phosphodiester-cleaving enzymes. However, if the phosphate group is modified to a thiophosphate the stability will be significantly increased; the resulting phosphorothioate (PS) oligos are much less susceptible to degradation. A result of the modification however is that the PS modified oligos will have a stereocenter at each modified phosphate group, leading to 2n-1 diastereomers. This means that for a 20-nucleotide long oligo there will be 219 = 524 288 species present, all of these with an individual retention time in the chromatography. The partial resolution of these species results in peak broadening, further complicating chromatographic separation. A way of resolving the problem of this peak broadening is to use ion-pairing agents with longer alkyl chains such as TBuAA for example. This will mask the thiophosphate chiral center, and almost eliminate the selectivity between the different diastereomers.
Figure 2 chromatograms show the effect of using TBuAA as ion-pairing agent, to achieve sharp peaks even with PS modified oligos. Phases used are Kromasil C4 with unmodified oligos and Kromasil Phenyl with fully phosphorothioated oligos
It can be seen in the Figure 3 chromatograms (zoomed-in portion of figure 2) that even with the phosphorothioated oligos it is possible to obtain very sharp peaks, despite the enormous number of different diastereomers.
All in all, these and further studies have indicated that Kromasil Phenyl is a very good choice when separating oligonucleotides in general, exhibiting very sharp peaks, and good selectivity.
For further details on the chromatographic conditions of these applications, please visit Kromasil website.
This study is published by courtesy of Dr. Martin Enmark, Karlstad University. The work was supported by the Swedish Knowledge Foundation for the KKS SYNERGY project 2016 “BIO-QC: Quality Control and Purification for New Biological Drugs” (grant number 20170059)
Digital Edition
Chromatography Today - Buyers' Guide 2022
October 2023
In This Edition Modern & Practical Applications - Accelerating ADC Development with Mass Spectrometry - Implementing High-Resolution Ion Mobility into Peptide Mapping Workflows Chromatogr...
View all digital editions
Events
May 05 2024 Seville, Spain
May 15 2024 Birmingham, UK
May 19 2024 Brno, Czech Republic
May 21 2024 Lagos, Nigeria
May 23 2024 Beijing, China