HPLC, UHPLC
Lactose determination based on the Chinese Pharmacopeia 2020 draft
Dec 10 2019
Background
Lactose has been used for many years by the pharmaceutical industry as an excipient [ref 1]. It is widely used in tablets, capsules, granules and freeze-dried products for various reasons such as a filler, a diluent, a binder and as a vehicle to assist in the delivery of the drug on interest. The Chinese Pharmacopoeia 2020 draft (ChP 2020 draft) indicates that the determination of lactose by chromatography needs to be carried out using sucrose as an internal standard [ref 2]. The pharmacopeia requires that the chromatographic resolution between lactose and sucrose should not be less than 1.5 when the compound concentration is 5mg/ml for each of them.
Briefly, lactose, C12H22O11, is a sugar that can be found in mammalian milk, a disaccharide derived from the condensation of two molecules: a glucose and a galactose. On the other hand, sucrose, is also a disaccharide with the same molecular weight as lactose ( CC12H22O11) but derived from the condensation of two molecules, glucose and fructose. Also, sucrose is naturally produced in plants. Lactose and sucrose structures are illustrated in figure 1.
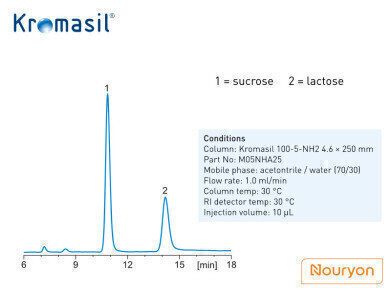
The work presented here deals with the chromatographic analysis of samples containing lactose and sucrose. Kromasil silica-based materials can be used when analysing sugars. Figure 2 shows the chromatographic result when employing a Kromasil 100-5-NH2 4.6 × 250 mm column to separate the two compounds. As seen in the figure the results meet the requirements stipulated by the ChP 2020 draft.
Figure 2 caption: Separation of sucrose and lactose on Kromasil NH2 according to ChP 2020.
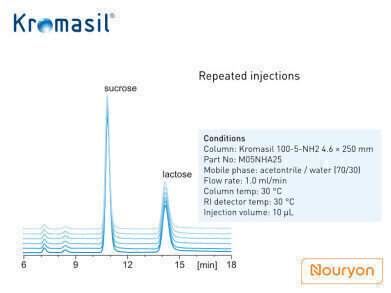
The chromatographic results corresponding to six repeated injections are shown in figure 3 using a Kromasil 100-5-NH2 4.6 × 250 mm column.
Standard sample preparation: Take appropriate amount of lactose and sucrose, precisely weighing and dilute with water to 5mg/ml for both lactose and sucrose. The chromatographic conditions are given in the figure. Standard analysis requirement: The resolution of lactose and sucrose should not be less than 1.5.
As seen in the figure, the reproducibility of the Kromasil NH2 column is excellent. In fact, the resolution of lactose and sucrose is much higher than 1.5, which meets and surpasses the requirements.
Figure 3 caption: Chromatogram of superimposed analytical results using the same column under the same chromatographic conditions and under the same sample concentration. Concentration of lactose and sucrose 5mg/ml.
Conclusion
This work shows that Kromasil 100-5-NH2 4.6 × 250 mm columns can be used for the determination of lactose according to Chinese Pharmacopoeia 2020 draft. It results in base line resolution for these compounds and shows excellent reproducibility.
This application can be found on the Kromasil website.
References:
[ref 1] Lactose, Evolutionary Role, Health Effects, and Applications (2019), Chapter 5- Application of lactose in the pharmaceutical industry, Pages 175-229.
[ref 2]: Chinese Pharmacopoeia.
Digital Edition
Chromatography Today - Buyers' Guide 2022
October 2023
In This Edition Modern & Practical Applications - Accelerating ADC Development with Mass Spectrometry - Implementing High-Resolution Ion Mobility into Peptide Mapping Workflows Chromatogr...
View all digital editions
Events
Apr 28 2024 Montreal, Quebec, Canada
May 05 2024 Seville, Spain
May 15 2024 Birmingham, UK
May 19 2024 Brno, Czech Republic
May 21 2024 Lagos, Nigeria